FDA Adopts Laissez-Faire Stance on Certain Health Wearables
FDA Commissioner Dr. Marty Makary joins ‘Varney & Co.’ to discuss the agency’s new approach to AI-powered health tools, wearable technology, and the future of medical innovation.
On Tuesday, FDA Commissioner Dr. Marty Makary made a significant announcement regarding the regulation of wearable devices. He stated that devices providing non-medical-grade information will not fall under FDA regulation. This move aims to clarify the agency’s stance on digital health and artificial intelligence.
During his appearance on “Varney & Co.“, Makary emphasized, “We want to let companies know, with very clear guidance, that if their device or software is simply providing information, they can do that without FDA regulation.” He added that the only exception would be if companies claim their products are medical-grade, such as providing clinically appropriate blood pressure measurements. “We don’t want people changing their medicines based on something that’s just a screening tool or an estimate of a physiological parameter,” he cautioned.
CHINA RACES AHEAD ON AI — TRUMP WARNS AMERICA CAN’T REGULATE ITSELF INTO DEFEAT
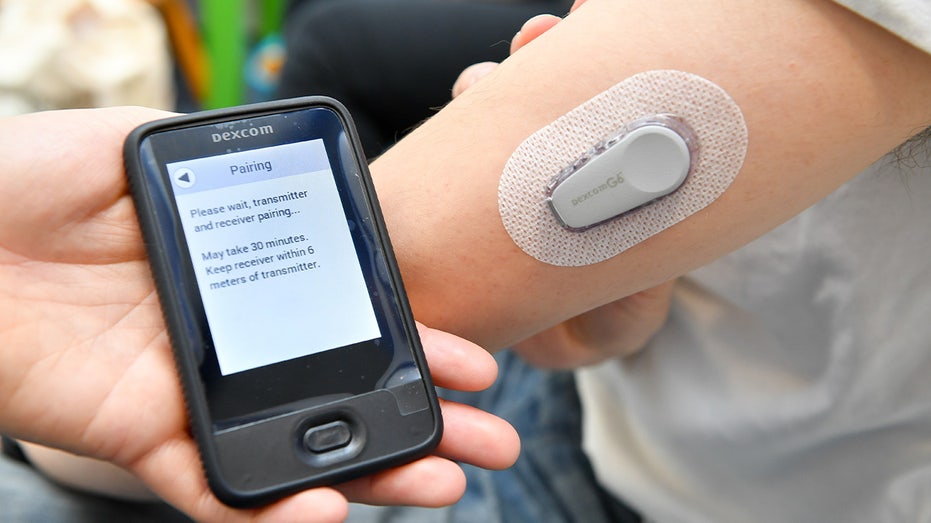
A Dexcom G6 Continuous Glucose Monitoring (CGM) System is fixed to a patient’s arm in a medical clinic based at University of Bristol on April 8, 2019. (Ben Birchall/PA Images via Getty Images / Getty Images)
This regulatory shift is designed to provide clearer guidance for the industry and enhance predictability for investors. Makary pointed out that as government agencies navigate the rapid advancements in artificial intelligence, the FDA must adopt a “proactive” stance.
When questioned about the accuracy of measurements from such wearables, Makary suggested that the market should determine this for non-medical-grade devices. “If they’re not making claims that they are medical grade, let’s let the market decide. Let’s let doctors choose from a competitive marketplace which ones they recommend for their patients,” he stated. He further explained that many AI medical devices and software technologies are continually improving, making it inappropriate for the FDA to apply outdated models to an evolving marketplace.
Additionally, Makary announced new guidance regarding support tools like Google and ChatGPT. He remarked, “If something is simply providing information like ChatGPT or Google, we’re not going to outrun that lion. We’re not going to go in there and say, ‘There’s one result that is inaccurate, therefore we’ve got to shut this down.'” He emphasized the need to promote these products while simultaneously guarding against significant safety concerns.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
HP CEO Enrique Lores discusses the use of AI in company technology on ‘The Claman Countdown.’
FDA Commissioner Dr. Marty Makary joins ‘Varney & Co.’ to discuss the agency’s new approach to AI-powered health tools, wearable technology, and the future of medical innovation.
On Tuesday, FDA Commissioner Dr. Marty Makary made a significant announcement regarding the regulation of wearable devices. He stated that devices providing non-medical-grade information will not fall under FDA regulation. This move aims to clarify the agency’s stance on digital health and artificial intelligence.
During his appearance on “Varney & Co.“, Makary emphasized, “We want to let companies know, with very clear guidance, that if their device or software is simply providing information, they can do that without FDA regulation.” He added that the only exception would be if companies claim their products are medical-grade, such as providing clinically appropriate blood pressure measurements. “We don’t want people changing their medicines based on something that’s just a screening tool or an estimate of a physiological parameter,” he cautioned.
CHINA RACES AHEAD ON AI — TRUMP WARNS AMERICA CAN’T REGULATE ITSELF INTO DEFEAT

A Dexcom G6 Continuous Glucose Monitoring (CGM) System is fixed to a patient’s arm in a medical clinic based at University of Bristol on April 8, 2019. (Ben Birchall/PA Images via Getty Images / Getty Images)
This regulatory shift is designed to provide clearer guidance for the industry and enhance predictability for investors. Makary pointed out that as government agencies navigate the rapid advancements in artificial intelligence, the FDA must adopt a “proactive” stance.
When questioned about the accuracy of measurements from such wearables, Makary suggested that the market should determine this for non-medical-grade devices. “If they’re not making claims that they are medical grade, let’s let the market decide. Let’s let doctors choose from a competitive marketplace which ones they recommend for their patients,” he stated. He further explained that many AI medical devices and software technologies are continually improving, making it inappropriate for the FDA to apply outdated models to an evolving marketplace.
Additionally, Makary announced new guidance regarding support tools like Google and ChatGPT. He remarked, “If something is simply providing information like ChatGPT or Google, we’re not going to outrun that lion. We’re not going to go in there and say, ‘There’s one result that is inaccurate, therefore we’ve got to shut this down.'” He emphasized the need to promote these products while simultaneously guarding against significant safety concerns.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
HP CEO Enrique Lores discusses the use of AI in company technology on ‘The Claman Countdown.’




